Based on customers' requirements, we provide plants for the manufacture of chemical synthesis pharmaceuticals and intermediates. Multi-purpose facilities are also possible. Our sealing technologies can also make a contribution in high potency pharmaceuticals. Our EPC solutions range from bulk powders to finished pharmaceutical products.

There are two main types of processes used to manufacture pharmaceuticals: chemical synthesis based on chemical reactions, and bioprocessing based on the ability of microorganisms and cells to produce useful substances. Chemical synthesis can be used to produce pharmaceutical products with relatively low molecular weights in large volumes in short timespans. In addition, various chemical modifications can be applied to enhance the activity of the substance produced.
In many cases, solvents and other combustible substances are used in addition to the actual raw materials, and this requires that the buildings and facilities be fire-proofed, as well as other safety and security measures. Also, in many cases, corrosive fluids are involved, requiring the use of glass linings or other anti-corrosive measures. The manufacturing processes often entail crystallization and crystal separation, with many processes needed for transport and insertion of solids. In general, pharmaceutical plants produce many different products, and production lines must be kept separate from one another to prevent cross-contamination of products. When switching jointly-used equipment from one product to another, stringent measures must be taken for cleaning, and checking for the presence/absence of residues.
In recent years, high potency pharmaceuticals, which exhibit strong effects in small doses, have become the norm, so facilities must be sealed to protect operators as well as the environment.
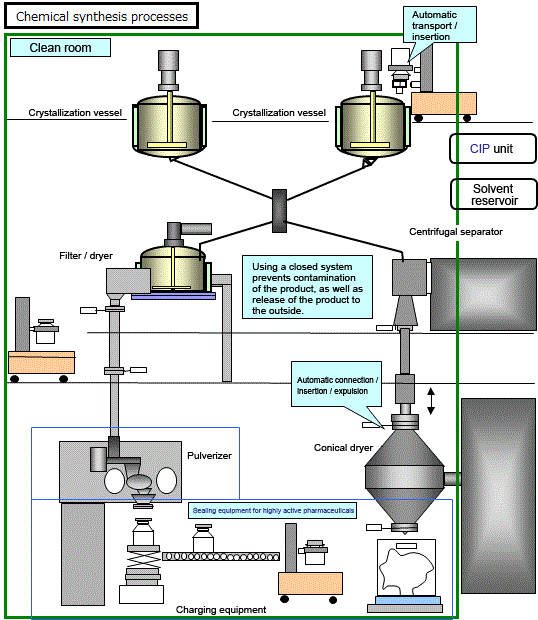
Manufacturing process for chemical synthesis pharmaceuticals
We can provide an EPC package combining engineering, procurement, construction and validation are possible.
Contact us from here.